In house, the one factor extra essential than guaranteeing entry to meals, water, and waste disposal (mixed!) is the necessity for a gradual provide of breathable air. The place the International Space Station (ISS) and different missions in Low Earth Orbit (LEO) may be resupplied repeatedly, missions working in deep house might want to produce their very own. There are a number of methods to go about this. A technique is to make use of bioregenerative life support systems (BLSSs), which make the most of photosynthetic organisms (like cyanobacteria) that soak up carbon dioxide and produce oxygen fuel and edible algae.
One other manner is to provide oxygen fuel from native sources, a course of often known as in-situ resource utilization (ISRU). This sometimes includes electrolysis, a course of through which electrical present is used to separate water molecules into hydrogen and oxygen fuel. Nonetheless, this course of is impractical for deep-space missions due to the quantity of vitality required, to not point out the truth that it doesn’t work properly in microgravity. In a recent study, a world group suggests an alternate technique that depends on a course of often known as magnetic part separation.
The analysis was led by Álvaro Romero-Calvo, a latest PhD graduate from the College of Colorado Boulder who’s presently an Assistant Professor on the Daniel Guggenheim School of Aerospace Engineering, a part of the Georgia Institute of Know-how. He was joined by researchers from the Center of Applied Space Technology and Microgravity (ZARM) on the College of Bremen, and the College of Warwick. The paper detailing their findings was printed on August 18th in Nature Chemistry.
 Mockup of the Oxygen Technology System Rack. Credit score: NASA
Mockup of the Oxygen Technology System Rack. Credit score: NASA
As famous, the present technique for producing oxygen in house is thru electrolysis, the place electrodes immersed in an electrolyte resolution (like salt) break up water molecules into oxygen and hydrogen. Aboard the ISS, that is accomplished by the Oxygen Generation Assembly (OGA), which spins water in a centrifuge to separate oxygen and hydrogen fuel bubbles from the liquid. In microgravity, nevertheless, fuel bubbles don’t float upwards, and people produced within the centrifuge have a tendency to stay suspended and stick with the electrodes. To extract them, a fancy fluid administration system is used that’s heavy, complicated, and occupies numerous house.
This makes such a system impractical since it could be very tough to tackle long-duration crewed missions. This was the conclusion reached by a group from NASA’s Ames Research Center who evaluated the OGA for crewed missions to Mars. Per the report, the group advisable that the OGA “for Mars ought to have decrease mass, higher reliability and maintainability, larger security, radiation hardening, and functionality for quiescent operation. NASA’s methodical, disciplined methods engineering course of needs to be used to develop the suitable system.”
To deal with this downside, the group proposed an alternate resolution that makes use of magnetism, which may make future life help methods lighter, less complicated, and extra sustainable. As Romero-Calvo mentioned in a Georgia Tech news release:
One might imagine that extracting fuel bubbles from liquids in house is so simple as opening a can of soda right here on Earth. Nonetheless, the dearth of buoyancy makes the extraction course of extremely tough, undermining the design and operation of oxygen manufacturing methods. On this paper, we show that two largely unexplored magnetic interactions – diamagnetism and magnetohydrodynamics – present an thrilling pathway to unravel this downside and develop different oxygen manufacturing architectures.
The group created their system utilizing off-the-shelf everlasting magnets, which they examined on the ZARM Drop Tower in Bremen – a 146 m (~480 ft) excessive tower that simulates microgravity. The group additionally developed a passive part separation system that collects the bubbles from the electrodes at designated spots. From this, they have been capable of show that magnetic fields can allow the separation of fuel bubbles from electrodes in a microgravity surroundings with out the necessity for heavy, complicated tools. The experiments additional confirmed that magnetic forces can enhance fuel bubble detachment and improve the effectivity of the electrochemical cells by as much as 240%.
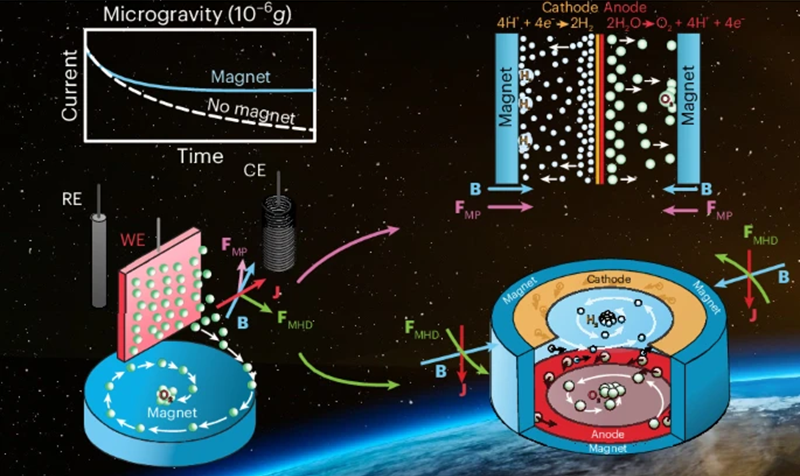 Diagram exhibiting the magnetically induced convection course of. Credit score: Nature Chemistry (2025)
Diagram exhibiting the magnetically induced convection course of. Credit score: Nature Chemistry (2025)
A key a part of this breakthrough is the 2 complementary approaches the group developed to gather oxygen bubbles from the electrode. The primary takes benefit of how water responds to magnets in microgravity by guiding fuel bubbles towards assortment factors, whereas the second employs magnetohydrodynamic forces. These forces come up naturally from the interplay between magnetic fields and electrical currents generated by electrolysis, making a spinning movement within the liquid that separates fuel bubbles from water via convective results.
This course of achieves the identical part separation as mechanical centrifuges, just like the one used aboard the ISS, but it surely makes use of magnetic forces as an alternative of mechanical rotation. “We have been capable of show that we don’t want centrifuges or any mechanical shifting components for separating the produced hydrogen and oxygen from the liquid electrolyte,” mentioned co-author Katerina Brinkert, an Honorary Professor on the College of Warwick and the Director of ZARM. “We don’t even want extra energy. As an alternative, it’s a fully passive, low-maintenance system.”
“Our group was capable of show that magnetic forces can feasibly management electrochemical bubbly flows in microgravity, departing from the state-of-the-art in low-gravity fluid mechanics and enabling future human spaceflight architectures,” Romero-Calvo added. The group’s work is the results of 4 years of joint analysis primarily based on Romero-Calvo’s unique concept, calculations, and numerical simulations. To validate the idea, Prof. Brinkert’s group at Warwick and ZARM developed the required experiments and gadgets to check the proposed technique in microgravity circumstances. As Dr. Shaumica Saravanabavan, a Ph.D. researcher on the College of Warwick, mentioned:
Throughout my journeys to ZARM, we confirmed the magnetic buoyancy impact for part separation in (photo-)electrolysis cells in a number of Drop Tower experiments, utilizing electrode supplies we partly fabricated at Warwick. I am proud to have contributed to advancing sustainable vitality applied sciences past Earth purposes.
This analysis presents alternatives for growing less complicated, cheaper, and extra sustainable life help methods for deep-space missions. Within the close to future, the group hopes to additional validate their technique by launching it to orbit, the place the system can function in microgravity for prolonged durations.
Additional Studying: University of Warwick, Nature Chemistry

